XenMD – Xenopus Modelling Disease XenMD for Patients and Families About us
XenMD Overview
XenMD offers a diagnostic service to clinicians with patients presenting in the South of England with a suspected rare monogenic disease and multi-systemic phenotype where a strong candidate variant of uncertain significance is identified and not easily assessed computationally or in vitro. Here, the Xenopus tropicalis model is used to assess loss-of-function gene changes and precisely model specific patient variants using ground-breaking gene-editing techniques.
XenMD: A pipeline for diagnosis of RGD in Xenopus tropicalis
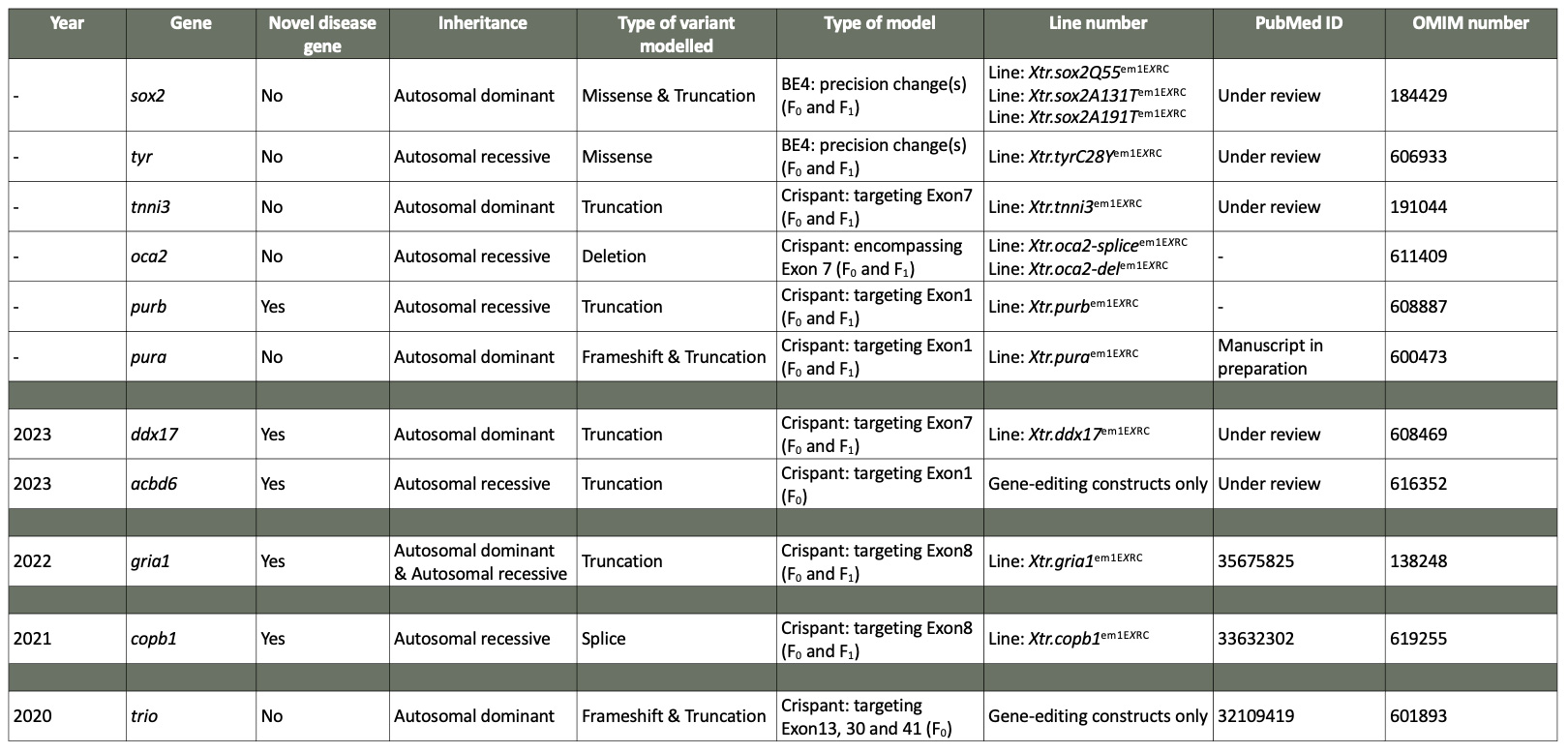
DNA sequencing has underpinned amazing improvements in diagnosis and treatment of the 1 in 20 people in the UK with a rare genetic disease (RGD). Despite this, a “diagnostic odyssey” continues beyond sequence analysis for 70% of patients. The bottleneck is gene function analysis, whereby syndromes affecting multiple organs require testing in vivo. Such analysis is unequivocally required to determine the variant-disease link with sufficient certainty to allow clinical intervention. In response the European Xenopus Resource Centre has driven Xenopus tropicalis, a diploid frog, to a prominent role in RGD diagnosis. This has been remarkably effective, with 23/30 patient gene variants of uncertain significance (VUSs) re-created successfully in tadpoles. These models have had immediate impact, directly informingpatient care and driving new requests for >100 VUS models from leading UK genetics centres.
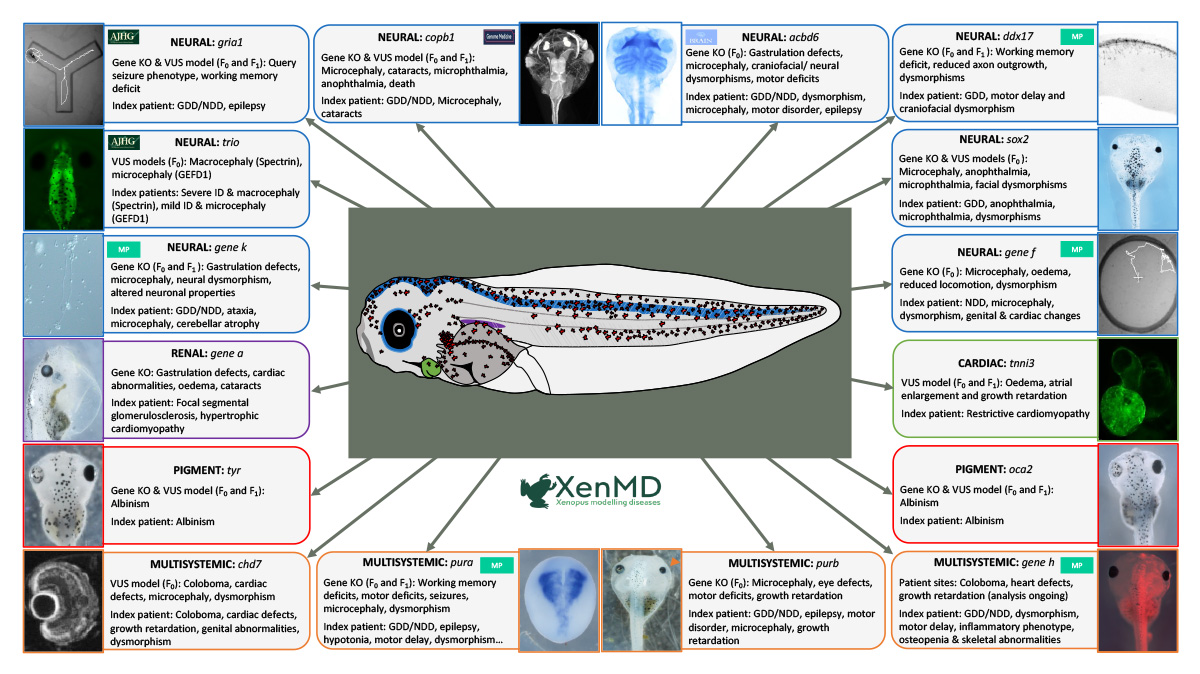
Figure 1 An overview of the phenotypic presentation of mutant Xenopus tropicalis following the application of CRISPR/Cas9 or BE4 base-editing techniques. Patient variants of uncertain significance (VUSs) have been re-created successfully in Xenopus tropicalis. In these models it was possible to measure comparable phenotypes to those observed in human patients in both the F0 and F1 generations.Novel, unpublished disease genes are represented as gene x. MP – Manuscript in preparation.
Gene changes in both F0 and F1Xenopus tadpoles reliably phenocopy key hallmarks of human disease
This type of analysis aims to produce a step change in the number of VUSs analysed. By prioritising VUSs with bioinformatic and clinical input we hope to maximise the impact on patient need. We currently use CRISPR/Cas9 to re-create frameshift, deletion and exon skipping VUSs in tadpoles and base-editors to make single nucleotide VUSs. Models are analysed by combination of omics, microscopy, staining, MicroCT, electrophysiology and quantitative behavioural assays.

Figure 2 An example phenotyping pipeline to assess novel disease-gene variants. A consistent in vivo phenotyping strategy for all gene variants, initially conducted experimenter blind, is essential to assess whether phenotypes observed in one ‘index case’ or individual are truly representative of each monogenic condition. By identifying the range of possible phenotypes produced by a gene variant this strategy not only enhances understanding of that gene in the context of disease but can also aid the discovery of other patients with the same condition.
acbd6 Case Study
Acyl-CoA-binding domain-containing protein 6 (ACBD6) is ubiquitously expressed, plays a role in the acylation of lipids and proteins, and regulates the N-myristoylation of proteins via N-myristoyltransferase enzymes. Whole exome sequencing has uncovered 43 affected individuals from 27 unrelated families with bi-allelic pathogenic, predominantly loss-of-function variants in ACBD6. The affected individuals typically present with a complex and progressive disease involving moderate-to-severe global developmental delay/intellectual disability, facial dysmorphism, neuro-anatomical abnormalities, movement disorders and behavioural abnormalities.
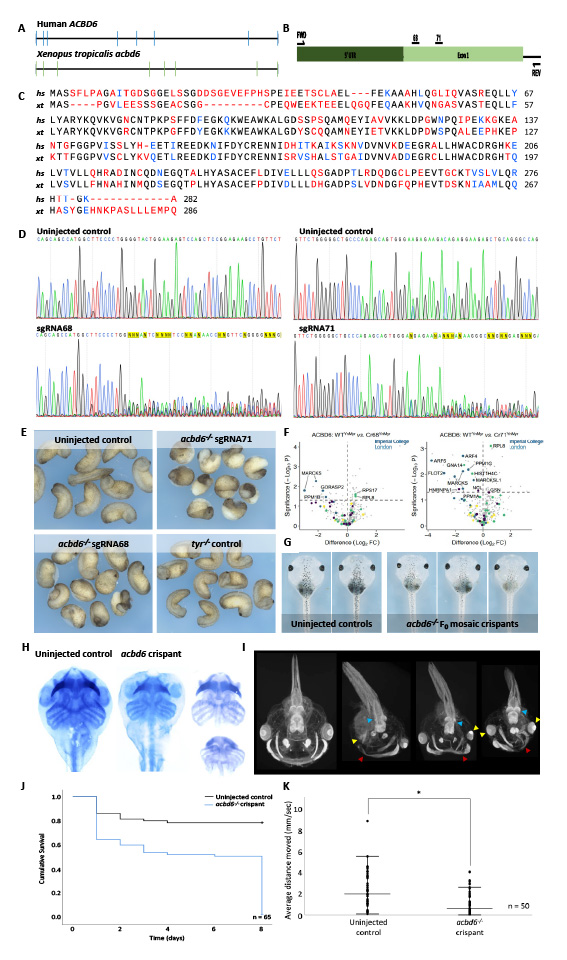
Figure 3 acbd6 Xenopus crispants have gastrulation, movement, craniofacial, brain and eye defects together with microcephaly. The gene structure of human (ACBD6) and Xenopus tropicalis (acbd6) reveals 8 exons (A). The partial X. tropicalis exon schematic depicts the target region within acbd6, exon1 and the relative position of the CRISPR/Cas9 sgRNAs and genotyping primers (B). The acbd6 amino-acid sequence of H. sapiens (NP_115736.1) and X. tropicalis (NP_001008065.2) aligned within SnapGene®5.2.4. using a local Smith-Waterman alignment shows modest sequence conservation (66.4%) (C). Sanger sequencing of the target region from uninjected and crispant embryos reveals both sgRNA-68 (GGACTCTTCCAGTACCCCAG) and sgRNA-71 (GGAGTGGGACGAGAAGACAG) result in consistent levels of CRISPR/Cas9 directed indel formation (ab1 trace files visualised in SnapGene®5.2.4.) (D). Gastrulation defects, including failure of blastopore closure and anterior posterior defects, were observed in F0 X. tropicalis embryos injected with two different CRISPR/Cas9 constructs (sgRNA-68 and sgRNA-71) disrupting exon 1 of acbd6 (E). Volcano plot comparing YnMyr labelling of proteins in wild-type and acbd6 crispant groups 68 & 71 show marked depletion of YnMyr-labelled proteins (proteins positioned on the left are reduced in crispants, whilst proteins positioned on the right are increased in crispants). The horizontal dotted line shows the significance threshold (FC: fold-change, W. Kallemeijn (p-value = 0.05)). (F) Those animals surviving to free-feeding stages presented with microcephaly, craniofacial dysmorphism and eye abnormalities (G). Alcian Blue staining marking the cartilaginous structures in the head and neck show equivalent structures between control and acbd6 crispant tadpoles revealing no gross morphological abnormalities (H). Detailed structural analysis in higher resolution MicroCT imaging (1% phosphotungstic acid contrast stain) revealed significant structural abnormalities in the facial musculature (Red arrows), abnormalities of the eye (microphthalmia, anophthalmia – Yellow arrows) and structural abnormalities in the brain most pronounced in the midbrain regions (Blue arrow) (I). The Kaplan-Meier survival analysis of 65 control and crispant tadpoles shows two periods of crispant-specific decline, the first at gastrula stages (day 0-1) and the second post-feeding (day 8, NF stage 47) (J). Locomotion analysis at NF44/45 revealed that crispants moved significantly less than control tadpoles (K).
Xenopus tropicalis and humans have the same acbd6 gene structures (Figure 3A) and share 66% amino acid identity (Figure 3C). To better understand the consequence of ACBD6 variants on human pathophysiology, we generated a Xenopus tropicalis acbd6knockout model (Figure 3B) using CRISPR/Cas9. We then characterised the role of ACBD6 on development, morphology, survival, locomotion and protein N-myristoylation. ICE analysis of target amplicon sequences (Figure 3D) showed that at gastrula stage 74% of alleles in the embryos had indels, and that 63.5% had a frameshift from a predominant 8bp deletion when using sgRNA 68. For sgRNA71 the average was a 53.6% knock-out from a mix of indels. The first notable phenotype was gastrulation failure due to reduced cell movements (Figure 3E). Comparing wild-type with acbd6 crispant 68 and 71 revealed a marked depletion of YnMyr-labelled proteins in the crispants, including all identified Xenopus proteins with co-translationally N-myristoylated human orthologues. Both crispants reveal prominent and significant reductions in proteins including MARCKS (which is required for normal cell movement during gastrulation in Xenopus), PPM1B, PPM1G (Figure 3F).
The embryos surviving past this point were noted to have significantly greater mosaicism and a greater proportion of in-frame frameshift mutations. At swimming tadpole stages more than half of the crispants had obvious craniofacial abnormalities (n = 36; Figure. 3G) and measurement of the head showed a decrease in average area from 2.07 +/- 0.36 mm2 in controls to 1.52 +/- 0.27 mm2 in crispants (t(34)=5.183, p<0.001). This was not a result of a defect in the structure of the head cartilage, although when in situ it did appear constrained by the overall head structure (Figure 3H (compare control and crispant)) and is consistent with the observed microcephaly. To detect more subtle changes in head structure, we compared control and crispant tadpoles by MicroCT (Figure 3I), revealing abnormalities in the location and structure of the eyes (yellow arrows), musculature of the face (red arrows) and structural abnormalities in the midbrain (blue arrows). After the deaths at gastrulation, crispants and control embryos survived similarly until feeding stage (Figure 3J). Whole proteome analysis followed by meta-analysis revealed upregulated proteins in crispants 68 and 71 were significantly affected in pathways of translation and metabolism, and notably, the ‘Parkinson-disease’-specific network was significantly enriched (data not shown). Comparing the movement of control and acbd6 crispant tadpoles showed the crispants to move less over a 10-minute period (average 2.37 mm/s for controls and 1.01 mm/s for crispants, n = 50, (t(78)=4.9, p=<0.001), Fig. 3K). These data came together with data collected in an ACBD6Zebrafish and human cell models to provide evidence that bi-allelic pathogenic variants in ACBD6 lead to a distinct neurodevelopmental syndrome accompanied by a complex and progressive cognitive and movement disorder.
XenMD Projects